Now, onto today's lesson on atoms and isotopes!
ATOMS
We all know that the atomic number is the number of protons in a single atom of an element. It is usually located in the top left of the element's square on the Periodic table. This tells you the atomic number (top left), the symbol (centre), the ion charge (indicated by a plus or minus sign on other periodic tables), and the atomic mass (top right). The atomic mass is only the average of all the isotopes of an element.

- Atomic Mass - Atomic Number = The Number of Neutrons
- (P + N) - (P) = (N)
- Here are two links that also explain this step in greater depth:
The most common isotope of hydrogen has no neutrons at all; there's also a hydrogen isotope with one neutron (deuterium) and two neutrons (tritium).
Examples:
- isotope: 54Fe --> mass number: 54 --> atomic number: 26 --> number of neutrons: 28
- isotope: 56Mn --> mass number: 56 --> atomic number: 25 --> number of neutrons: 31
- isotope: 237Np --> mass number: 237 --> atomic number: 93 --> number of neutrons: 144
- isotope: 14C --> mass number: 14 --> atomic number: 6 --> number of neutrons: 8
ISOTOPE NOTATION
The atomic number is written as a subscript on the left of the element symbol. The mass number is written as a superscript on the left of the element symbol. Finally, the ionic charge (if any) appears as a superscript on the right side of the element symbol.
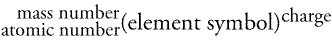
Let's look at hydrogen:

You'll notice that the smallest isotope, hydrogen-1, has only one neutron. Deuterium and Tritium each have more neutrons. Also, the nucleus does not repel itself, because the neutrons act as spacers.
A Mass Spectrometer is a device that can be used to determine the relative abundance and the mass of the isotopes of elements. To deflect a beam of charged particles, this device uses a charged field. The lightest particles will bend the most. We can measure where the particle lands on the screen, and in what abundance. Here is a diagram of a mass spectrometer:

We know that there are "preferred" combinations of neutrons and protons, at which the forces holding nuclei together seem to balance best. In fact, light elements tend to have about as many neutrons as protons, while heavy elements apparently need more neutrons than protons in order to stick together.
Let's say we found the following information:
And we want to find the average atomic mass. The 5 peaks in the mass spectrum shows that there are 5 isotopes of zirconium - with relative isotopic masses of 90, 91, 92, 94 and 96. So, we know:
- zirconium-90 51.5
- zirconium-91 11.2
- zirconium-92 17.1
- zirconium-94 17.4
- zirconium-96 2.8
(51.5 x 90) + (11.2 x 91) + (17.1 x 92) + (17.4 x 94) + (2.8 x 96) = 9131.8. Then, we take 9131.8 and divide by 100. The average mass of these 100 atoms would be 9131.8 / 100 = 91.3 (to 3 significant figures). Find zirconium in the periodic table, and 91.3 is approximately the relative atomic mass of zirconium. Yay! Next class, period tables and trends!

No comments:
Post a Comment