FOUR ELEMENT THEORY (ARISTOTLE)
When things catch fire, moisture is released, air can be felt coming up from it, and ashes show the earth that it contains.

This theory states that there are four elements that make up everything → Earth, Fire, Wind, and Water. It simplified our view of the world, but can these types of matter be broken down even further?
DEMOCRITUS’ THEORY

However, this is only a conceptual model. There is no mention of a nucleus, or its constituents. Aristotle and the Greek philosophers did not accept Democritus’ theory. What holds these particles together? Why doesn’t matter fall apart?
LAVOISIER’S THEORY
The Law of Conservation of Mass states that the mass of an isolated system remains the same. Therefore matter cannot be created or destroyed.

PROUST’S THEORY
The Law of Constant Composition states that a chemical compound always contains exactly the same proportion of elements by mass. For example, the mass of water is always 88.9% oxygen and 11.1% hydrogen.
DALTON’S THEORY
Dalton concluded that the properties of matter could be explained in terms of atoms:
- Each element is composed of extremely small particles called atoms
- All atoms of a given element are identical, but they differ from those of any other element
- Atoms are neither created nor destroyed in any chemical reaction
- A given compound always has the same relative numbers and kinds of atoms
- Atoms are solid, indestructible spheres (and look kind of like billiard balls). Different spheres exist for different elements. His theory is also based on the law of conservation of mass.


This theory does not mention subatomic particles, however.
J. J. THOMSON’S THEORY
He created the raisin bun model, and said that solid positive spheres have negative particles in them. He proposed the first atomic theory that includes protons (positive), and electrons (negative). Thomson demonstrated that electrons existed with a cathode ray tube – a vacuum tube in which a stream of electrons is produced and directed onto a fluorescent screen (e.g. in a television or visual display unit, creating images and text).

RUTHERFORD’S THEORY
He showed that all atoms have a positive, dense center with electrons outside of it. The famous gold foil experiment:
He showed that all atoms have a positive, dense center with electrons outside of it. The famous gold foil experiment:
He also suggested that atoms are mostly empty space and he explains why electrons spin around the nucleus. His model is a planetary model.
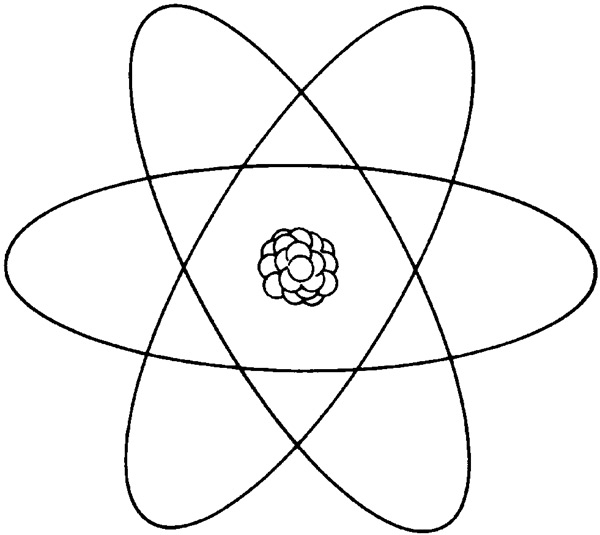
BOHR’S THEORY
The advent of quantum mechanics. He applied quantum theory to Rutherford's atomic structure by assuming that electrons travel in stationary orbits defined by their angular momentum. We’ll learn more about Bohr later on in the unit.
Here’s a song about atomic theory:
Next class is our unit 1 test!

No comments:
Post a Comment