Before representing Bohr's theory, you need to know that all atoms are electrically neutral, which means that they have the same number of protons and electrons. You should also know the two ways to represent Bohr's theory; the energy level model and the Bohr model. Electrons occupy shells which are divided into orbitals:
-2 electrons in the first shell
-8 electrons in the second shell
-8 electrons in the third shell
The last two shells are octets, and all atoms want stable octets (a full number of electrons in the last shell). The fourth shell holds 18 electrons. An ion will have a charge that is positive (less electrons than protons) or negative (more electrons than protons).
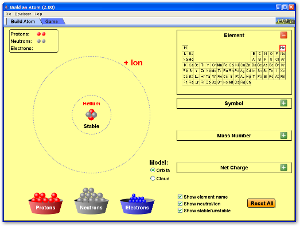
You can build an atom yourself, from scratch, here:
Here is an energy level model for Argon:
If you look at the periodic table, you will notice that the mass of Argon is 39.95 or 40 (which is the number on top). Its atomic number (the number of protons in the nucleus) is 18 (which is the number on the bottom). These numbers, along with the chemical symbol, Ar, make up what is called atomic notation. Argon happens to be a noble gas, so its shells are full (this is why noble gases do not react easily). Because atoms are neutral, the number of electrons is the same as the number of protons (18 in this case). Keeping in mind that there are 2 electrons in the first shell, 8 electrons in the second, and 8 electrons in the third, we can demonstrate that there are 18 electrons in total by writing 2e, 8e, and 8e above the atomic notation. In chemistry the energy level model is used more because the Bohr model doesn't represent the shape of the orbitals (each pair of electrons) correctly and is therefore incorrect (we'll learn more about these shapes later on!).
BOHR MODEL
Here is a bohr model for Argon:
We draw dots to represent the electrons. Notice how there are 2 electrons in the first shell, 8 electrons in the second, and 8 electrons in the third, making a total of 18 electrons. Thus, the atom is neutral. Also, notice that the electrons are drawn in pairs. We draw 1 electron in each orbital before we start pairing them up.
Each row in the periodic table corresponds to the number of shells in a Bohr diagram. For example, there are 3 shells in the bohr diagram of Argon, because Argon is in the 3rd row of the periodic table. The periodic table group (vertical column) determines the number of valence electrons (8; in this case, a stable octet). The number within the unit's place identifies how many valence electrons are contained within the elements listed under that particular column. Helium, (2 valence electrons) and Groups 3-12 (transition metals) are an exception to this rule.
Some atoms have charges (ions). In the example below there is a Fluorine atom which has a charge of -1 because it needs just one more valence electron (electrons in the outer most shell) to have a stable octet, and there is a Calcium atom that has a +2 charge because it needs to get rid of 2 valence electrons to have a stable octet.

Next time, we will investigate Quantum Theory!




No comments:
Post a Comment