DRAWING DOT DIAGRAMS
The atomic symbol represents the nucleus. In order to know how many electrons one will need to draw, one will need to determine how many valence electrons that atom has. Electrons are drawn as dots and placed in four orbitals each containing a maximum of 2e- around the atomic symbol. Each orbital gets 1e- before they get paired with the left overs electrons.
Examples:
Examples:

LEWIS DIAGRAMS FOR COMPOUNDS AND IONS
In bonds that are covalent, meaning a bond between two non-metals, electrons are shared.
1. First one must find the number of valence electrons for "x" atom in the molecule.
2. Draw the atoms so that they will fill each orbital which means that one needs to place one in each orbital before one begins pairing up.
Example:
-CH4
The two blue-boxed diagrams are Carbon and Hydrogen by themselves and the white boxed one is the compound:
The two blue-boxed diagrams are Carbon and Hydrogen by themselves and the white boxed one is the compound:



DOUBLE AND TRIPLE BONDS
In some cases the only way a covalent bond can be drawn is if they share more than one electron.
Examples:

IONIC COMPOUNDS
In ionic compounds electrons transfer from one element to another. One must determine the number of valence electrons on the cation (Mr. Doktor has a good way of remembering that a cation is positive; think of a cat scratching out a plus sign). Then, one must move these to the anion. Write the charges outside of the brackets.
Examples:
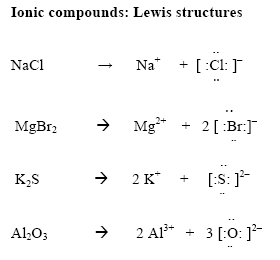
Here are two helpful websites that also explain Lewis diagrams:
And here is a video to help explain Lewis Diagrams visually:

No comments:
Post a Comment