An "ester" is an organic, often fragrant compound formed in a reaction between an acid and alcohol with the elimination of water. In today's class, we learned all about these esters.
The COO group connects two hydrocarbon chains in an ester:
To name an ester:
- The hydrocarbon chain attached directly to che carbon side of the COO group has its "e" ending changed to "-oate." The C in the COO group is considered to be part of the parent hydrocarbon chain.
- The hydrocarbon chain attached to the oxygen side of the COO group is named as an alkyl group; the name of the alkyl group is used as a separate, initial word.
Esterfication:
Esterfication is the reaction of a carboxylic acid and an alcohol to form an ester and water! They are prepared by reacting in the presence of an inorganic acid (eg. HCl). The odour of an organic acid is a "sharp, pungent, and biting" smell. Alcohols also have a "sharp" odour. The smell of methanol and ethanol has very little odour but it tends to "catch" in the nasal passage. Propanol and higher alcohols have a very intense often unpleasant odours which also "catches" in the nasal passage. However, the odour of esters is generally very pleasant (fortunately). They form the basis of many fragrant fruit and flower smells! For example:
-Pentyl ethanoate: banana odour
-Octyl ethanoate: orange rind
-Pentyl propanoate: apricot odour
-Ethyl methanoate: rum odour
As always, it's time for some examples!
1. What is the structural diagram for...
a)Methyl butanoate?

b)Butyl methanoate?
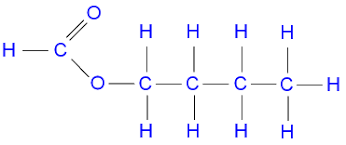
2. Name the following esters:
a)
Answer: Ethyl propanoate
b)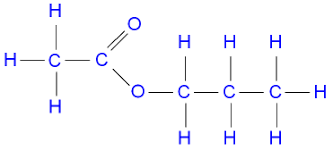
Answer: Propyl ethanoate
3. What esterfication forms the ester METHYL BUTANOATE? (Draw the structural diagrams)

Here's an awesome video related to this lesson:
1. What is the structural diagram for...
a)Methyl butanoate?

b)Butyl methanoate?
2. Name the following esters:
a)

Answer: Ethyl propanoate
b)
Answer: Propyl ethanoate
3. What esterfication forms the ester METHYL BUTANOATE? (Draw the structural diagrams)

Here's an awesome video related to this lesson:



No comments:
Post a Comment