We had a very short class to experiment with hydrates, as it was post-secondary day. It was quite a fascinating experiment.
Recall that a hydrate is a compound that can form lattices which can bond to water molecules. These crystals contain water inside them which can be released by heating. In other words, hydrates are ionic compounds that contain an inorganic salt compound loosely bound to water. The hydrate we used was cobalt(ous) chloride hexahydrate.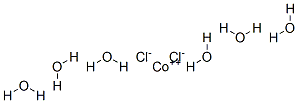

PURPOSE
The purpose of this experiment was to determine the anhydrous (with no water molecules attached) mass of the hydrate and compare this with the actual mass of water that should be present.
MATERIALS
For this experiment, we used a Bunsen burner, a test tube, a test tube rack, a test tube clamp, a weight scale, and a hydrate (cobaltous chloride hexahydrate). For safety reasons, we wore lab coats, rolled up our sleeves so that they would not catch on fire, and wore safety goggles. We felt like we were professional chemists.
PROCEDURE
Our goal was to boil away all of the water so that only the inorganic salt compound was left. We followed the following procedure:
- Find the mass of the empty test tube, using the weight scale (this is so we can determine the mass of the hydrate by itself).
- Fill the test tube with about one centimetre of the hydrate.
- Find the mass of the test tube and the hydrate, using the weight scale.
- Using extreme caution, connect and light the Bunsen burner. The gas flow should be adjusted so that it is about five centimetres tall. Always assume that the Bunsen burner is on!
- Using the clamps, pick up the test tube and carefully hold it in above the Bunsen burner.
- Gently heat the test tube by moving the test tube in and out of the flame for about five minutes or until all the water has boiled away.
- Find the mass of the test tube and the inorganic salt compound, ensuring none of the chemicals inside spill.
It is very important that you do not leave the hydrate in the flame for too long, because the anhydrous solution will begin to react with oxygen.
OBSERVATIONS
- We noticed that the initial mass, compared to the final mass, was greater than the final mass. This is because the water evaporated, and less matter was present in the test tube after heating it with the Bunsen burner.
- While heating the substance, a color change was evident; the substance changed from magenta, to a dark purple, to a light purple, and finally, to a light blue.
- We could hear that a gas was being released. This is the water evaporating.
-chloride-hexahydrate-sample.jpg)
_chloride.jpg/220px-Cobalt(II)_chloride.jpg)
ANALYSIS
After the experiment, we determined how much water was released during heating, and what percent of the hydrate was water.
To determine how much water was released during heating, you must first find the mass of the substance on its own, since you weighed both the test tube and the substance inside. So, subtract the mass of the empty test tube from the mass you obtained before heating. This is the mass of the substance on its own, before heating. To find the mass of the substance on its own, after heating, subtract the mass of the empty test tube from the mass you obtained after heating. Now that you have the mass of the substance before and after, you can find out how many grams of water were released during heating, by subtracting the mass at the end of the experiment from the mass at the beginning of the experiment.
To determine what percent of the hydrate was water, divide the amount of water by the mass of the substance at the beginning of the experiment and multiply by 100. In our case, 45% of the hydrate was water. Because the actual percent of water in this hydrate IS 45%, our experiment had 0% error.
If you do not know how to calculate percent error, please refer to the September Post: Measurement and Chemistry! The formula for calculating percent error is:

CONCLUSION
We now have a much better understanding of the composition of a hydrate. Here is a video on the experiment we carried out:
Next class is our chapter test on atomic theory!

No comments:
Post a Comment